Bottom
We recently constructed a Bacillus subtilis strain (CCTCC M 2016536) from which we deleted the srfC, spoIIAC, nprE, aprE, and amyE genes. This strain is capable of robust recombinant protein production and amenable to high cell density fermentation. Since the promoter is among the factors influencing target protein production, optimization of the initial promoter, PamyQ from Bacillus amyloliquefaciens, should improve protein expression with this strain. This study was carried out to develop a new high-level expression system in Bacillus subtilis Recombinant CTCCC M 2016536.
Methods
- Bacterial strains and media
Bacterial strains used in this study are described in Additional File 1: Table S1 Escherichia coli JM109 was used as the host for gene cloning and plasmid construction. Bacillus subtilis CCTCC M 2016536 was used as a host for reporter protein expression. LB medium consisted of 10 g/l tryptone, 5 g/l yeast extract and 10 g/l NaCl. TB medium contained 12 g/L tryptone, 24 g/L yeast extract, 5 g/L glycerol, 12.54 g/L K2HPO4, and 2.31 g/L KH2PO4.
The medium used to increase β-CGTase production in the 3 L fermentor was a modified mineral salt medium, consisting of 20 g/L yeast extract, 2 g/L Na2SO3, 30 g/L L corn steep powder, 10 g/L glucose, 1 g/L MgSO4 7H2O, 1 g/L (NH4)2–H-citrate, 2.68 g/L (NH4)2SO4, 4 g/L NaH2PO4 H2O, 14.6 g/L K2HPO4, and 3 mL/L trace element solution. The feed solution consisted of 40 ml/l trace element solution, 500 g/l glucose, 63.36 g/l (NH4)2HPO4 and 7.89 g/l MgSO4·7H2O.
- Shake flask culture conditions
For routine plasmid construction, E. coli JM109 was incubated in LB medium supplemented with 100 mg/L ampicillin for 10 h at 37 °C with shaking at 200 pm. To express β-CGTase in shake-flask fermentation, plasmid strains B. subtilis CTCCC M 2016536 transformed with the appropriate plasmids were incubated in 10 mL of LB medium supplemented with 20 mg/L tetracyclines for 10 h at 37 °C with shaking at 200ºC rpm.
A portion (2.5 mL; 5% [v/v]) of this overnight culture was used to inoculate 50 mL of TB medium containing 20 mg/L tetracyclines, which was then incubated for 48 h at 30 °C. with stirring at 200 rpm. . The culture was harvested by centrifugation at 12,000 xg for 10 min at 4°C to obtain culture supernatant, which contained indicator proteins.
- β-CGTase activity
β-CGTase cyclization activity was measured by adding 0.1 ml of crude enzyme suitably diluted with 2 ml of preheated 1% (w/v) soluble starch dissolved in 25 mM phosphate buffer (pH 5.5) and then incubating the mixtures at 50 °C. C for 10 min. The reaction was stopped by adding 0.2 mL of 0.6 M HCl and incubating for an additional 5-10 min.
At this point, 0.5 mL of 0.6 M Na2CO3 and 0.2 mL of 1.2 mM phenolphthalein were added sequentially and the mixture was kept at room temperature for 15 min to allow the colour to develop. Finally, the absorbance of the test solution at 550 nm was measured using a spectrophotometer (BioPhotometer plus, Eppendorf Co., Hamburg, Germany). One unit of β-CGTase was defined as the amount of enzyme that formed 1 μmol of β-cyclodextrin per minute from soluble starch under the conditions.
- α-CGTase activity
α-CGTase cyclization activity was measured using an assay similar to that described above for β-CGTase activity. An aliquot of 0.1 ml of the appropriately diluted crude enzyme was added to 2 ml of prewarmed 2% (w/v) soluble starch dissolved in 25 mM phosphate buffer (pH 5.5). The resulting mixture was incubated at 50 °C for 10 min. The reaction was stopped by adding 0.2 mL 3M HCl and incubating for an additional 5-10 min.
At this point, 0.2 mL of 0.44 mM methyl orange was added and the mixture was kept at 16 °C for 15 min to allow the colour to develop. Finally, the absorbance of the test solution at 505 nm was measured using a spectrophotometer. One unit of α-CGTase was defined as the amount of enzyme that formed 1 μmol of α-cyclodextrin per minute from soluble starch under the conditions.
- Pullulanase activity
Pullulanase activity was measured using a reaction mixture prepared by adding 0.1 ml of the suitably diluted crude enzyme to a preheated mixture of 1 ml of 1% (w/v) pullulan dissolved in deionized water and 0.9 ml of 50 mM sodium acetate buffer (pH 4.5). The resulting mixture was incubated at 60 °C for 10 min.
The reaction was stopped by adding 3 mL of the 3,5-dinitrosalicylic acid solution, boiling for 7 min, and then immediately immersing the reaction in an ice-water bath. Finally, 10 mL of deionized water were added and the absorbance of the reaction mixture was measured at 540 nm. One unit of pullulanase activity was defined as the amount of enzyme that liberates 1 μmol of reducing sugars per minute from pullulan under the conditions.
- Determination of bacterial biomass
To determine dry cell weight, 5 mL culture samples were centrifuged at 12,000 × g for 10 min at 4 °C. These precipitates were resuspended in a 0.9% (w/v) NaCl solution and then centrifuged again at 12,000 xg for 10 min at 4 °C. The resulting precipitates were dried at 105 °C to constant weight.
- SDS-PAGE analysis of reporter proteins
Culture supernatants containing reporter proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12.5% separating gel. 20 ul of supernatant was mixed with 5 μL of sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer (5x), after boiling in a water bath for 5 min, 8 μL of the mixture was added to the sodium dodecyl sulfate-polyacrylamide gel. . Protein bands were stained with 0.25% Coomassie Brilliant Blue R-250.
- Statistic analysis
All experiments were performed independently in triplicate. Data are presented as mean ± standard deviation. Statistical analyzes were performed using Student’s t-test and differences resulting in P < 0.05 were considered statistically significant.
Results
Using the enzyme β-cyclodextrin glycosyltransferase (β-CGTase) as the reporter protein and B. subtilis CTCCC M 2016536 as the host, nine plasmids equipped with unique promoters were examined by shake flask culture. The plasmid containing the PamyQ’ promoter produced the highest extracellular β-CGTase activity; 24.1 U/mL. Subsequently, six plasmids equipped with dual promoters were constructed and evaluated using this same method.
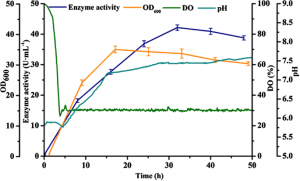
The plasmid containing the PHpaII-PamyQ′ dual promoter produced the highest extracellular β-CGTase activity (30.5 U/mL) and was relatively repressed by glucose. The PHpaII-PamyQ’ dual promoter also mediated substantial expression of extracellular pullulanase (90.7 U/mL) and α-CGTase (9.5 U/mL) during shake-flask culture, demonstrating the general applicability of this system. Finally, the production of β-CGTase was investigated using the PHpaII-PamyQ′ double promoter system in a 3 L fermentor. The extracellular expression of β-CGTase reached 571.2 U/mL (2.5 mg/mL), which demonstrates the potential of this system for use in industrial applications.
Conclusions
The dual-promoter PHpaII-PamyQ’ system was found to support the superior expression of extracellular proteins in B. subtilis CCTCC M 2016536. This system appears to be of general application and can be extended.